My Favorite Chemical Reaction
(Or so I thought…)

I decided to investigate liquid hand soap and the foaming reactions….
!!!Spoiler Alert!!!
The foaming action is NOT a chemical reaction at all. Instead, it’s just a change of structure. In true Scientific fashion, I made a quick pivot and started at square one, the process of saponification (soap making). This is for bar soap but don’t worry, I threw in some videos for liquid soap and foaming info for fun.
Saponification Reaction
Saponification Reaction
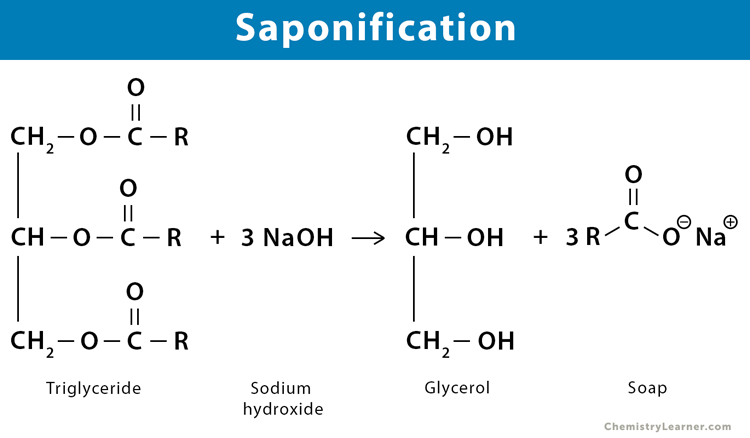
Saponification is the chemical reaction that occurs when a fat or oil reacts with a base to produce glycerol (glycerin) and soap (a salt of a fatty acid). This reaction is essential for creating the soap that eventually gets converted into foam when dispensed.
The Process

1. Soap Production:
- Ingredients: The primary ingredients for saponification are triglycerides (fats and oils) and a strong base (commonly sodium hydroxide, NaOH, or potassium hydroxide, KOH).
- Reaction: When triglycerides react with the base, they break down into glycerol and soap molecules.
2. Foaming Mechanism:
(only used for liquid soap)
- Physical Action: The foaming pump on the soap dispenser aerates the liquid soap, creating foam. This is a physical process where the liquid soap is mixed with air.
- Surfactant Action: The soap molecules act as surfactants, reducing the surface tension of water and trapping air to form bubbles, allowing the formation of a stable foam.

Balanced Chemical Equation
fat/oil + sodium hydroxide → glycerol + fatty acid salt (soap)

Soap
C3H5(OOCR)3 (l)+ 3NaOH (aq) → C3H5(OH)3 (aq)+ 3RCOONa (aq)
- Reactants: Triglycerides (fats/oils) and Sodium Hydroxide
- Products: Glycerol and Soap (sodium salt of fatty acids)
For Liquid soap you use KOH (Potassium Hydroxide) instead of Sodium Hydroxide (NaOH)
Type of Reaction
The saponification reaction is a hydrolysis reaction, specifically a base-catalyzed ester hydrolysis. It is also classified as an acid-base reaction because the base (NaOH) reacts with the ester (triglyceride).
Explanation
Hydrolysis: This is because water (from the hydroxide ion) breaks the ester bonds in the triglyceride.
Acid-Base: The hydroxide ion (OH-) acts as a base, attacking the carbonyl carbon in the ester bond of the triglyceride, leading to the formation of glycerol and fatty acid salts.
Takeaway

Before this assignment, I thought I was paying for a premium soap, but now I understand I’m just overpaying for a dilution of soap that looks and smells pretty. Bath and Bodyworks has been taking my money for years!!! LOL
I feel much more confident in understanding how to safely attempt making a soap that is more natural and better for my sensitive skin.
It all started with a question, how does my fancy soap go from a liquid to foam. It ended with me going back to the beginning and learning about the reactants in order to achieve soap. Pretty neat experience for me.
Make your own
Super easy DIY soap making… Give it the college try!!!
If you choose to make Liquid Soap here is a very detailed D.I.Y video for you.
Here is a quick D.I.Y. tutorial I found on youtube that well help you make your own and save a ton of money by diluting inexpensive liquid hand soap and getting more for less.