The Atom
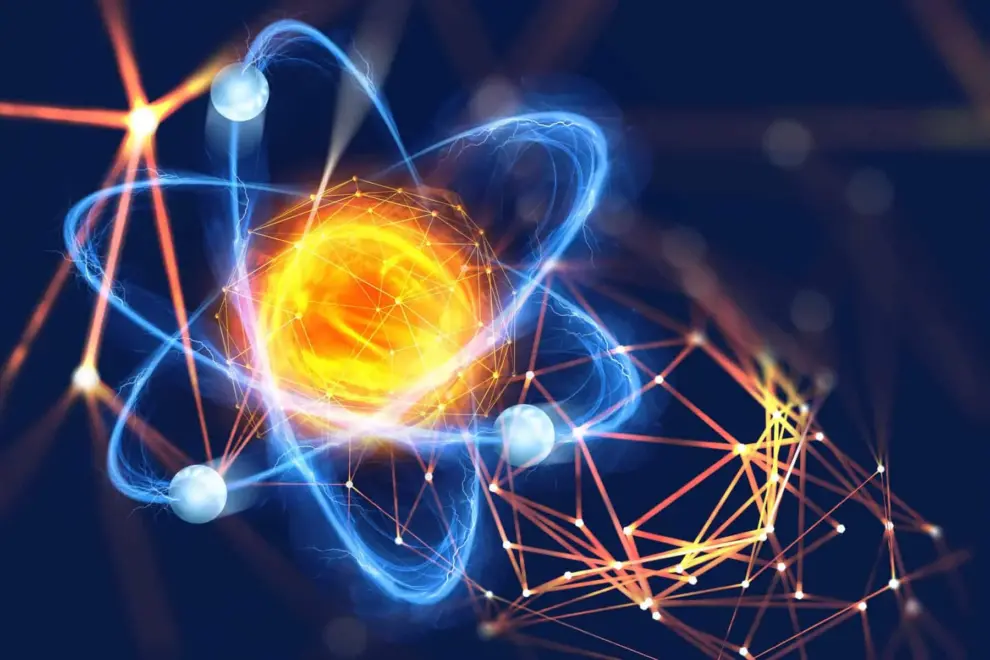
Defined: An Atom is the smallest piece of an element that maintains
the identity of that element.

Structure of an Atom
Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus.
- Electrons have a negative ( – ) charge.
- Protons have a positive ( + ) charge.
- Neutrons have no charge (they are neutral)
- The Nucleus consists of electrically positive protons and electrically neutral neutrons.
Shells
The electron shells of an atom are populated from the inside out, with electrons filling up the low-energy shells closer to the nucleus before they move into the higher-energy shells further out.

The Periodic Table
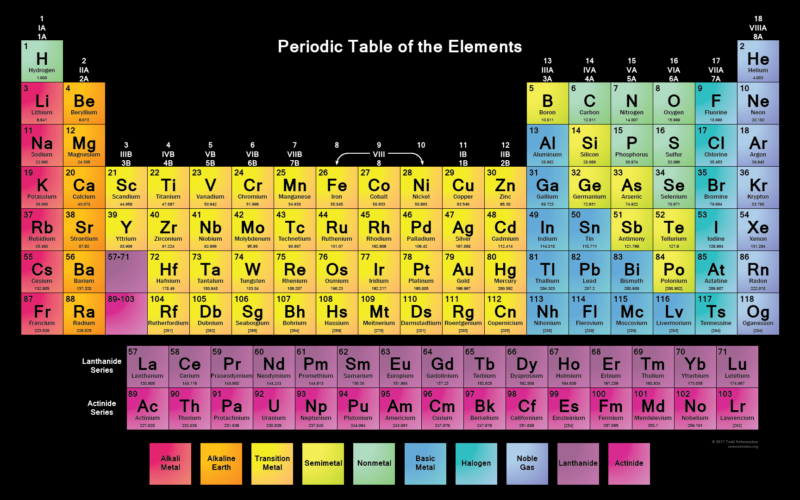
As you move along each row (or period), there are repeating patterns in the chemical and physical properties of the elements in each one. For example, you’ll find metals on the left-hand side and non-metals on the right.
the element
Periodic tables generally display two numbers with each element. The smaller number is the atomic number. This is the number of protons, which is unique to each element and doesn’t change. The larger number is the relative atomic mass of an element – the higher the number, the greater its mass.

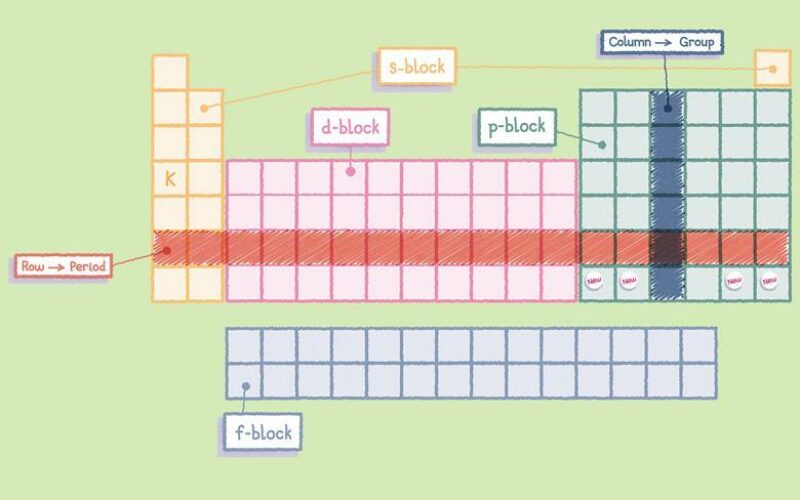
The Orbit
Each block is named after its characteristic orbital: s-block, p-block, d-block, f-block and g-block.
Electron Configuration chart
This chart is used to illustrate the order in which electrons are filled in atomic orbitals.
According to this principle, electrons are filled in the following order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…

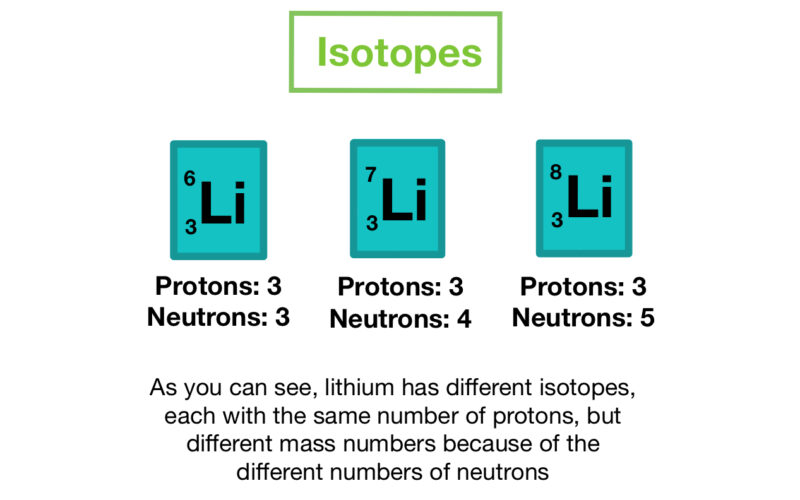
Isotopes
An isotope is a variation of the same element with a different number of neutrons in their nucleus.
- Stable isotopes have nuclei and they are non-radioactive.
- Unstable isotopes have unstable nuclei and they are radioactive.