Chemical Reactions

Chemical Reactions: are the processes by which chemicals interact to form new chemicals with different compositions.

Chemical Equation
A representation of a chemical reaction using symbols of the elements to indicate the amount of substance, usually in moles, of each reactant and product.
Balancing
- Reactants are starting materials and are written on the left-hand side of the equation.
- Products are the end-result of the reaction and are written on the right-hand side of the equation.
In order make both sides equal, you will need to multiply the number of atoms in each element until both sides are equal.
- Coefficient is a number placed in front of a chemical symbol or formula that shows how many atoms or molecules of the substance are involved in the reaction.

Precipitation Reaction
Occur when cations and anions in aqueous solution combine to form an insoluble ionic solid called a precipitate.
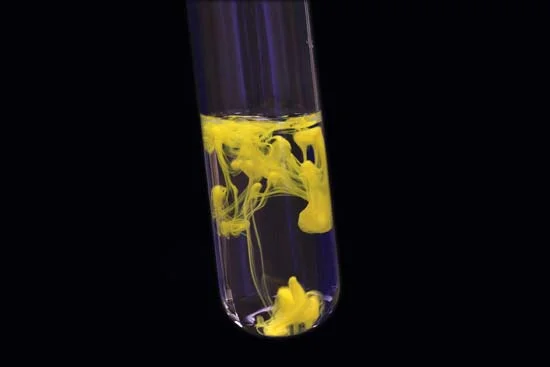
This reaction is both a precipitation reaction as well as double displacement reaction.
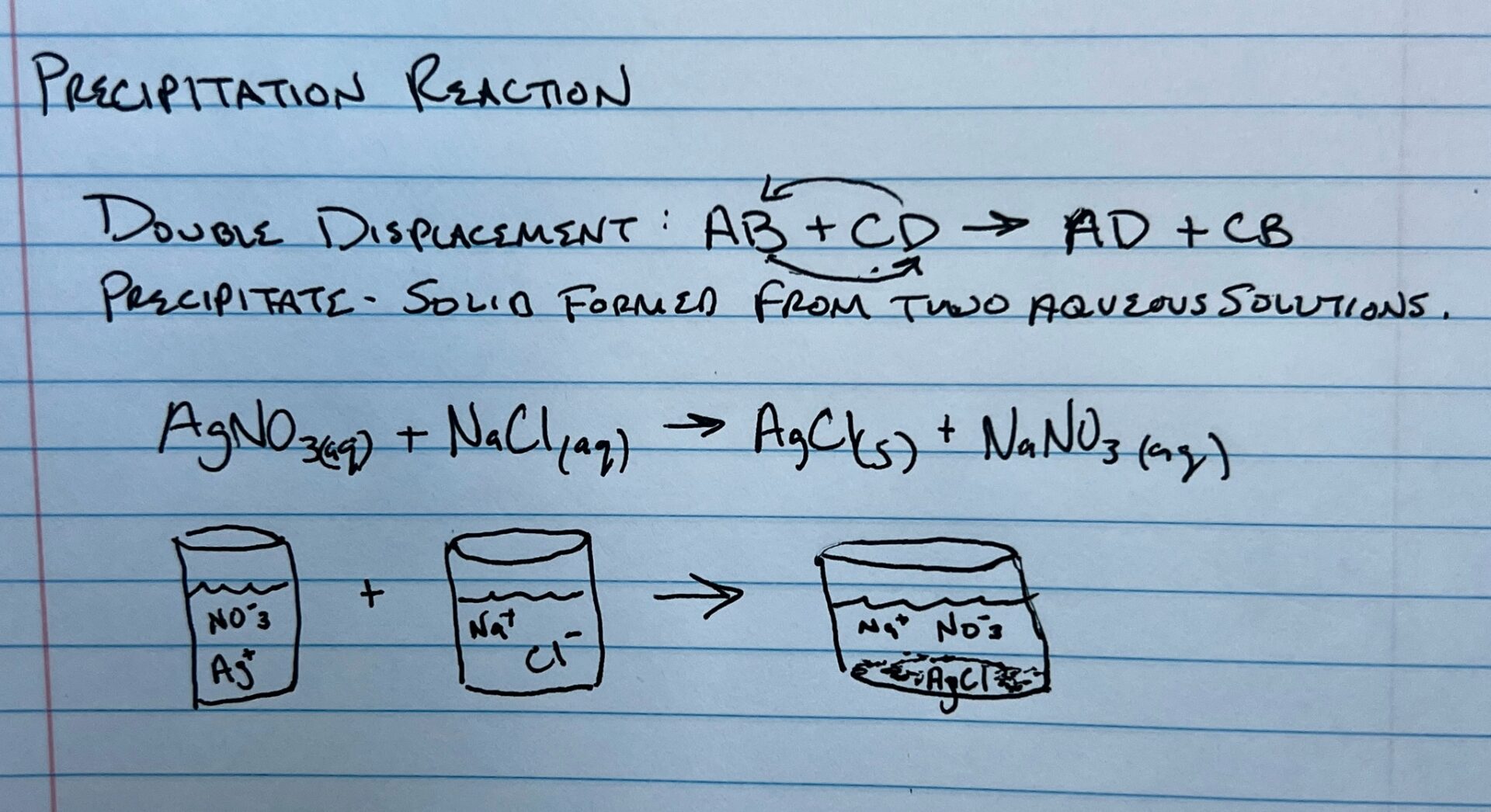
In a double displacement reaction the anions are exchanged and in a precipitation reaction, a precipitate is formed in the end. In this case, AgCl is the precipitate.
Oxidation-Reduction Reactions
is a type of chemical reaction in which the oxidation states of a reactant change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state.


Neutralization Reaction
A neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH– ions to generate water.
- Base + Acid -> Salt + Water
- HCl + NaOH -> H20 + NaCl (Salt)
- HNO3 + KOH -> H2O + KNO3 (Salt)
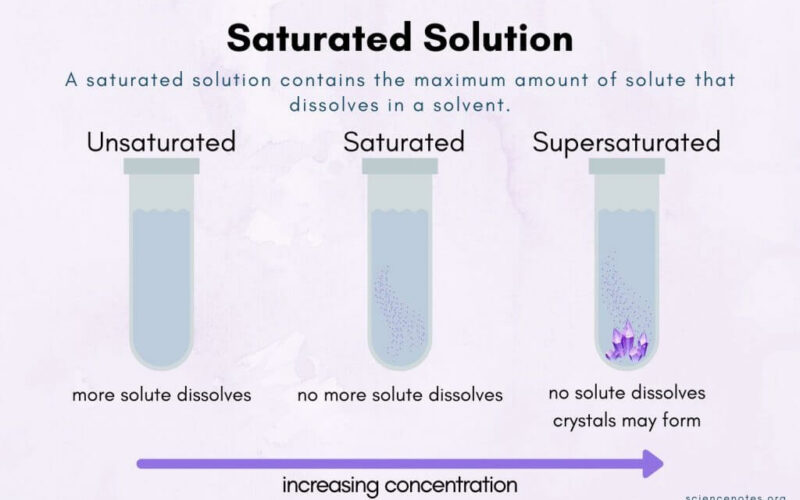
Solubility Rules
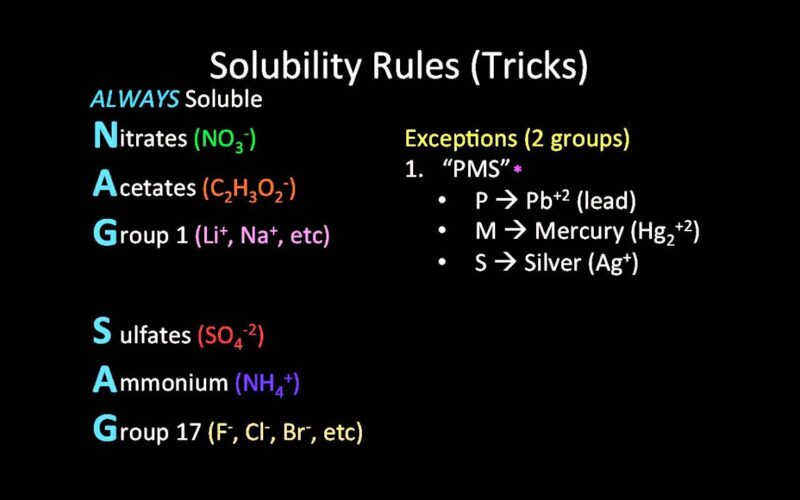
Solubility is the ability of a solid, liquid, or gaseous chemical substance (solute) to dissolve in a solvent and form a solution.
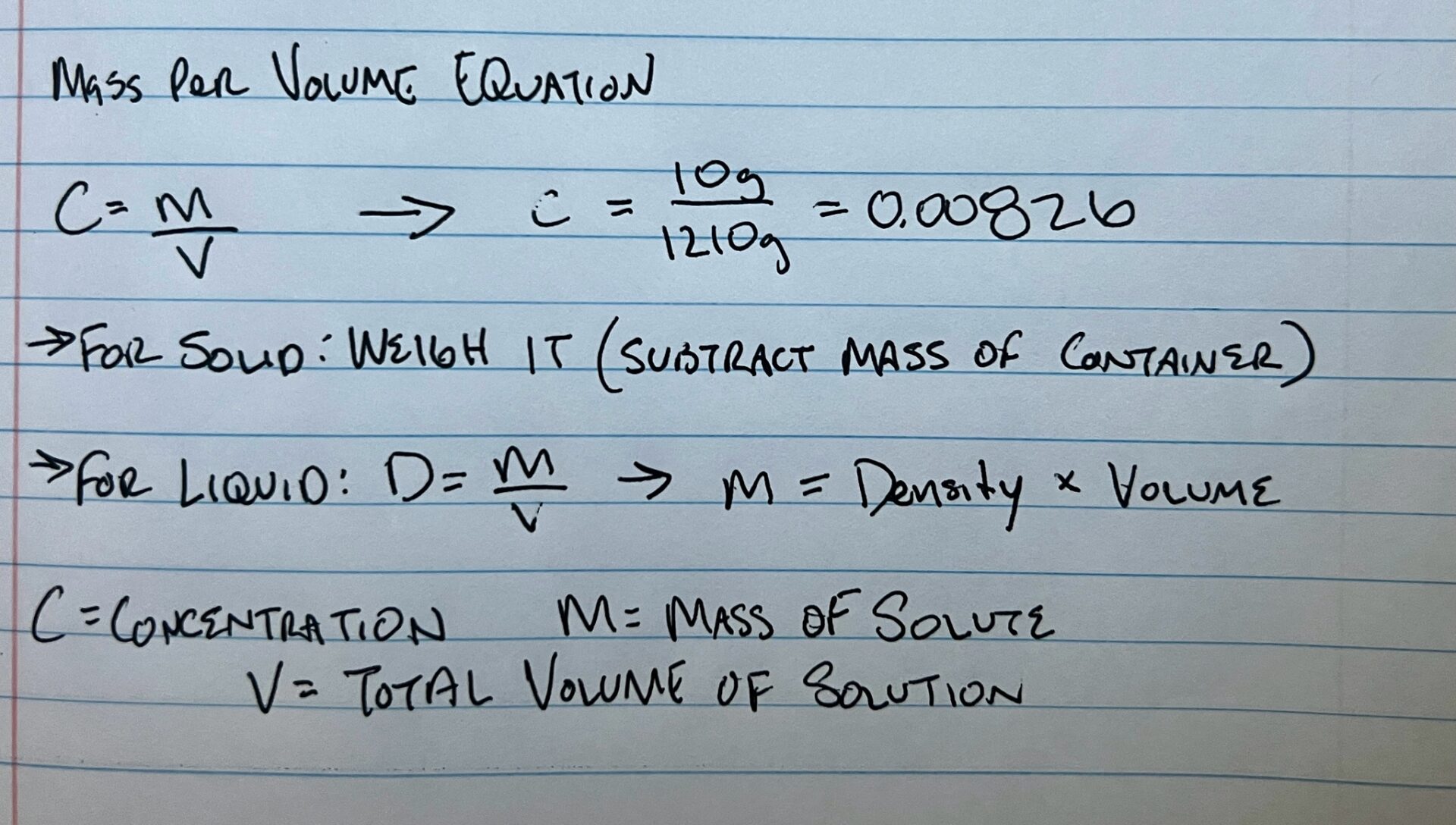
Concentration of Solute
Divide the mass of the solute by the total volume of the solution. Write out the equation C = m/V, where m is the mass of the solute and V is the total volume of the solution. Plug in the values you found for the mass and volume, and divide them to find the concentration of your solution.